Your Guide to the Proposed National Drug Code Format Changes [2022]
The FDA proposes a rule that would revise the National Drug Code format. What does this mean, and how may it impact you? Read on to find out.

What is the Proposed Rule?
On July 22, 2022, the FDA announced a proposed rule, Revising the National Drug Code Format and Drug Label Barcode Requirements (Docket No. FDA-2021-N-1351). The goal is to establish a 12-digit format for all National Drug Codes assigned by the FDA and allow drug product barcode labels to use either linear or nonlinear barcodes.
What is the Current National Drug Code?
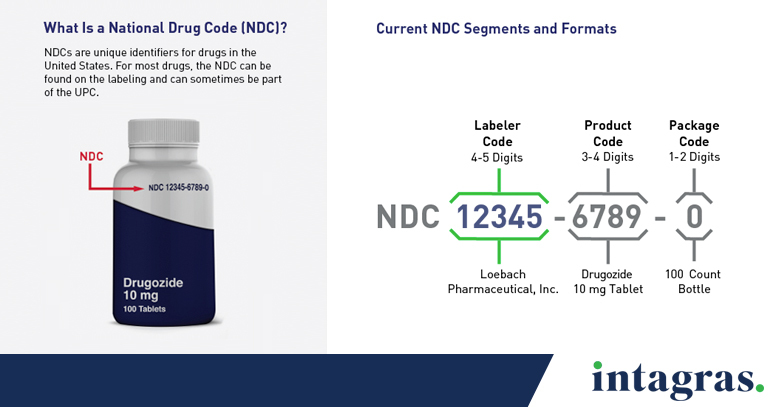
Every drug marketed in the United States is required to have a unique National Drug Code (NDC). Currently, NDCs can be 10 or 11 digits long and vary in format. Each code has three segments in this order: a labeler code, product code and package code.
Labeler Code
The labeler code is the first segment of 4-5 digits that indicates the establishment or labeler.
Product Code
The product code is the second segment of 3-4 digits that represents a product or group of products.
Package Code
The package code is the third segment of 1-2 digits that describes the package sizes and types.
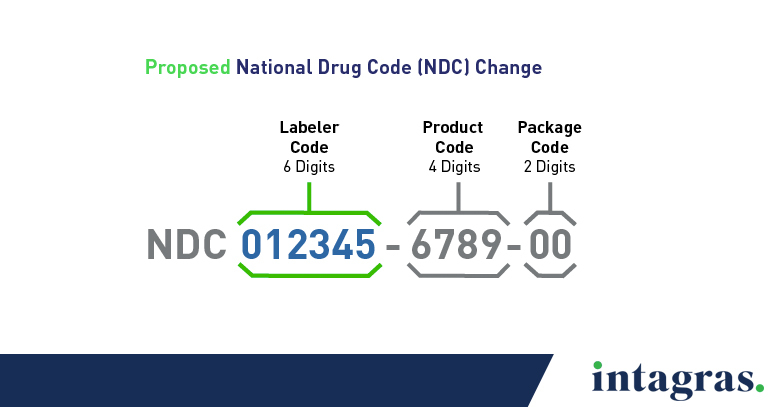
What is the Proposed National Drug Code?
The FDA is proposing every NDC be 12 digits long with three specific segments and one uniform format. The order of the segments would stay the same, but the number of digits per segment would be different. Here’s how it would add up:
- Labeler code: 6 digits
- Product code: 4 digits
- Package code: 2 digits
Why is the Proposed Change Happening Now?
The FDA is running out of 10-digit NDCs because they issued NDCs at a much faster rate during the COVID-19 pandemic. Therefore, the FDA is working to increase the number of digits to future-proof the system.
Who Would Be Impacted By the Change?
Anyone in the healthcare system who uses NDCs will be affected by the proposed change, including:
- Dentist offices
- Drug databanks
- Federal agencies using the NDC
- Hospitals
- Human and animal drug manufacturers
- Importers
- Insurers and payors
- Nursing care facilities
- Pharmacies
- Prisons
- Small clinics and healthcare practitioners
- State and local governments
- Supply chain stakeholders
- Wholesale distributors
This change would require you to develop and implement a system that creates 12-digit NDCs for Standard Product Labeling (SPL) files instead of the current 10-digit codes. Luckily, the FDA is giving you time to adjust and make the appropriate changes before mandating the 12-digit codes.
When Would the Change Happen?
Final Rule Publishes
The FDA proposes an effective date five years after the publication of the final rule. This would provide you time to update your systems.
Effective Date
The final rule will include an effective date. Once that’s established, the FDA will start assigning new NDCs in the uniform 12-digit format. However, the FDA won’t require you to resubmit all your existing drug listing files. Instead, the FDA plans to convert existing NDCs on the effective date by adding leading zeros to any segments that don’t have the correct number of digits. This saves you time and money.
Transition Period
After the effective date, there will be a three-year transition period. During this time, the FDA recommends you start using the 12-digit NDCs at the earliest opportunity. However, the FDA will accept 10-digit NDCs if they were assigned before the effective date. To avoid confusion, the FDA will also maintain and publish both 10-digit and 12-digit NDCs for specific drugs.
How Can You Prepare for the Change?
Once the FDA announces the effective date, you should start working on updating your systems so they work with 12-digit NDC files. Various integrated systems throughout the pharmaceutical supply chain interact, from ERP systems to eCTD systems. They all need to be updated to handle 12-digit codes so they can be ready to run when the rule change goes live. You should also ensure your system can work with both linear and nonlinear barcodes.
If you use SaaS-based systems, you should check to ensure those platforms will be updated and ready for the effective date.
One of the benefits of updating your systems for 12-digit NDCs is the automation of manual tasks. For example, you often have to manually update NDCs when moving data from one system to another. With a uniform NDC length, all of these tasks will be able to be automated.
Conclusion
If the FDA’s proposed rule change goes into effect, all new NDCs must follow a structured 12-code format. While this change would affect the entire healthcare industry, the FDA is giving you time to adjust your systems so they can be ready on Day 1.
Need regulatory submission help? Talk to us.
Have questions? Need more info? Talk to one of our experts or request a demo of our SPL Portal today.